Broad-spectrum Antivirals
Developing safe, potent therapeutics that neutralise a wide range of viral threats through direct inactivation strategies.
Platform Overview
We focus on the discovery and development of broad-spectrum antivirals that can inactivate multiple viruses on contact. Our goal is to create flexible therapeutic platforms capable of responding rapidly to known and emerging threats.
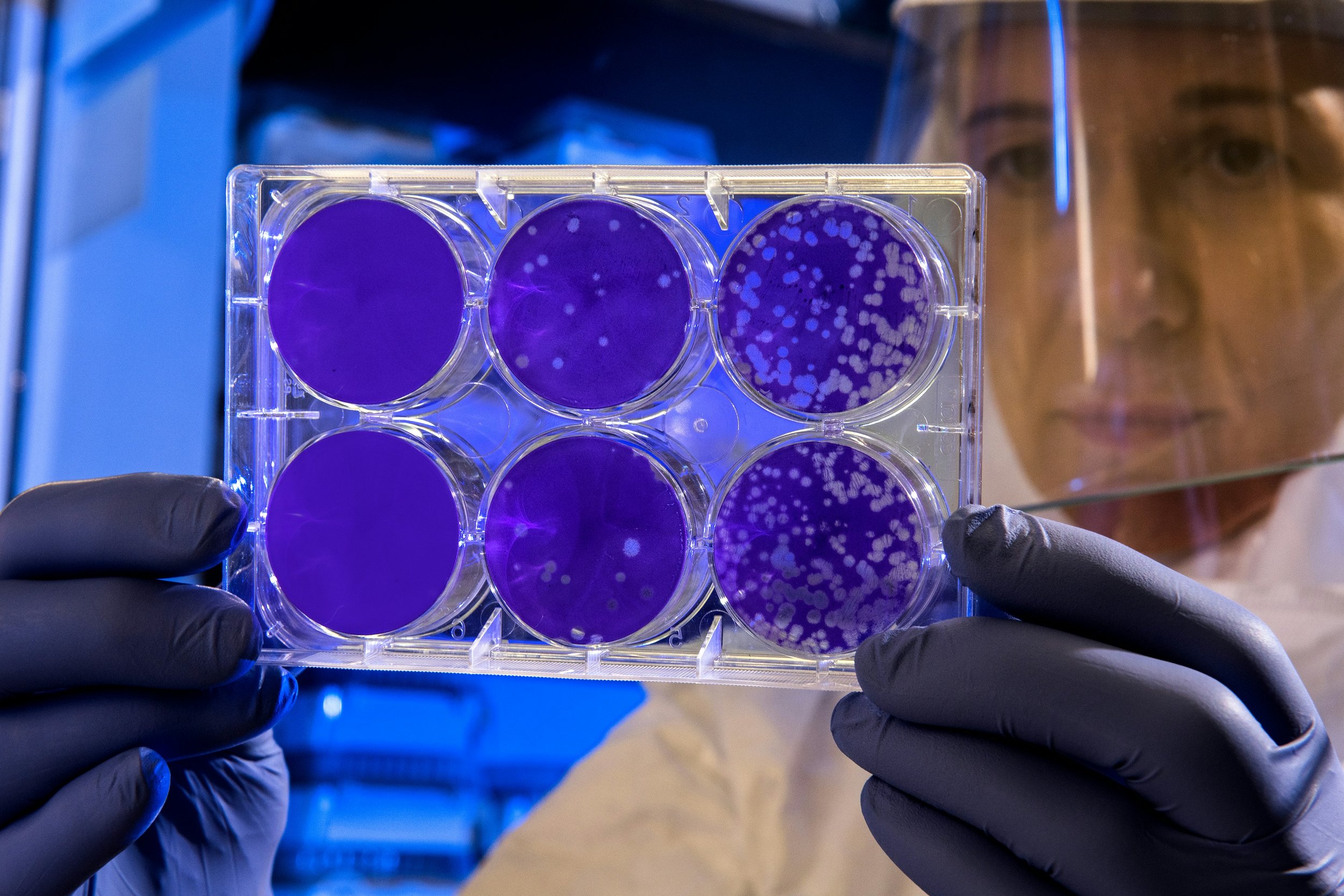
We achieve this by developing biocompatible virucidal antivirals with broad-spectrum activity. Our materials have shown potent inhibition (including in vivo) across a wide range of viruses including HSV-1, HSV-2, Lentivirus, HPV, Hepatitis C, RSV, PIV3, HMPV, Human coronaviruses, Enteroviruses, Dengue 1 & 4, Chikungunya and Zika. Current work focuses on expanding viral coverage and collecting of pre-clincal data for future studies.
Mechanisms of Action
Virucidal Disruption
compounds that directly inactivate viral particles
Fusion Inhibitors
blocking viral membrane fusion with host cells
Replication inhibitors
compounds that block viral replication intracellularly
Antivirals
At present only a small subset of human viral diseases have approved drugs. Dengue, rotavirus (Diarrhea), Respiratory syncytial virus (RSV), Ebola, Zika, Influenza, and many others do not have drugs. The approved drugs almost exclusively have antiviral action, which is they prevent infection by blocking viral replication intra-cellularly. Such antivirals tend to loose effectiveness upon viral mutations, are all virus specific, and -because of their intracellular mechanism- have some intrinsic associated toxicity. There are extracellular mechanisms to fight viral diseases, i.e. virustatic and virucidal.
Virustatic vs Virucidal
Virustatic drugs inhibit viruses by reversibly binding to them outside the cell, blocking entry. Heparin—a naturally occurring polymer best known as an anticoagulant—is one example. It can bind viral receptors and prevent infection, but only temporarily. Many heparin-inspired materials show similar broad-spectrum, non-toxic effects. However, because binding is reversible, these drugs lose efficacy when diluted in bodily fluids, allowing viruses to regain infectivity—limiting their medical relevance.
Schematic representation of virustatic mechanism
Virucidal drugs irreversibly inactivate viruses outside the cell, making them a powerful strategy for treating infections and limiting resistance. While many virucidal agents exist—like surfactants, bleach, acids, and bases—they’re too toxic for therapeutic use. The challenge is harming the virus without harming the host, since both share similar chemistry. A true breakthrough would be a broad-spectrum, non-toxic virucidal drug—something virustatics lack in efficacy and current virucidals lack in safety.
Schematic representation of virucidal mechanism
Our Advancement
We have discovered a set of design rules that allow for the synthesis of a range of materials with broad-spectrum virucidal properties, while remaining biocompatible. Our seminal work in this area has identified polymer architecture as a key parameter to obtain such virucidal properties. Through our work on creating star polymer antivirals we have discovered a completely new antiviral mechanism that is highly effective and destroy virions on contact, in vitro and in vivo.
Electron microscopy image of virus before (left) and after (right) interaction with our virucidal antivirals

